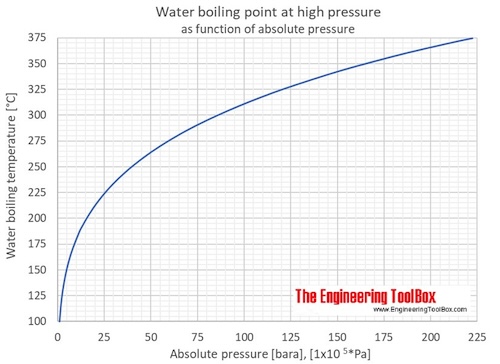
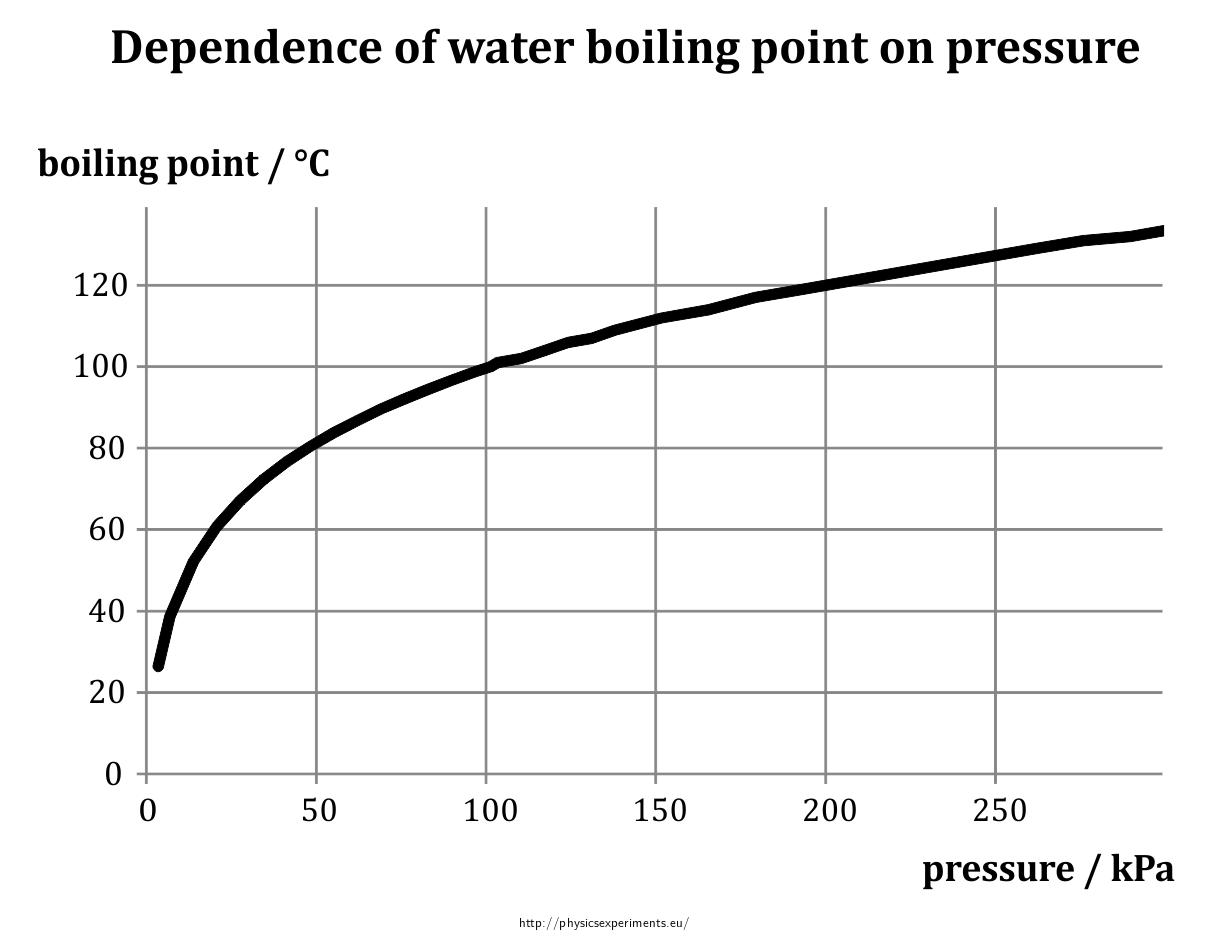
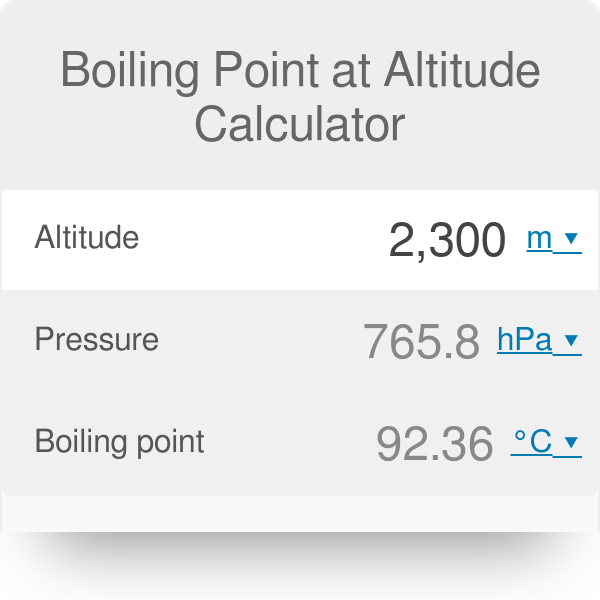
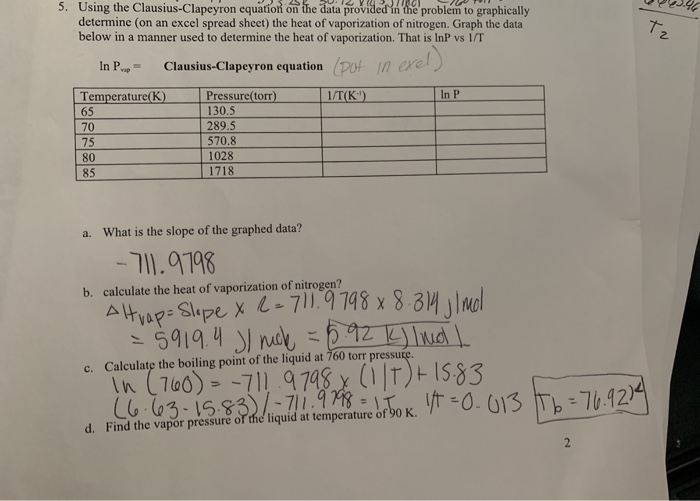
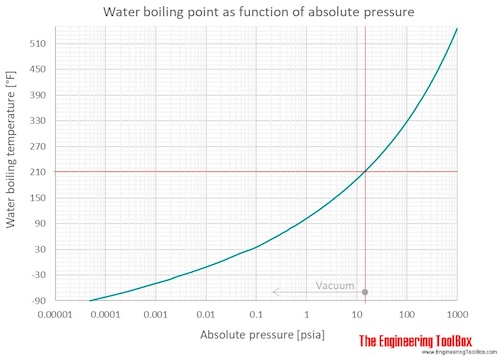
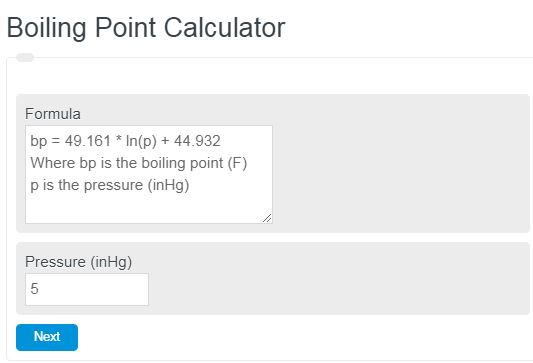
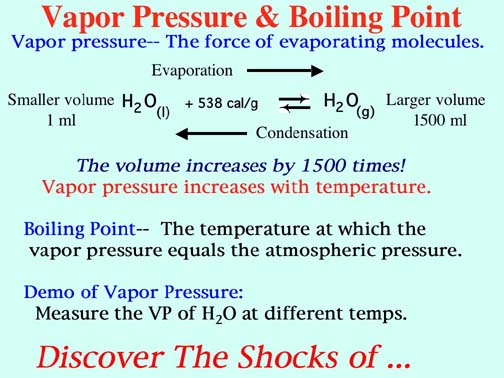
SOLVED:Suppose the vapor pressure of a substance is measured at two different temperatures. (a) By using the ClausiusClapeyron equation, Equation 11.1, derive the following relationship between the vapor pressures, P_{1} and P_{2},

Methanethiol has a vapor pressure of 429 torr at −25 ∘c and a normal boiling point of 6.0 ∘c. find - Brainly.com


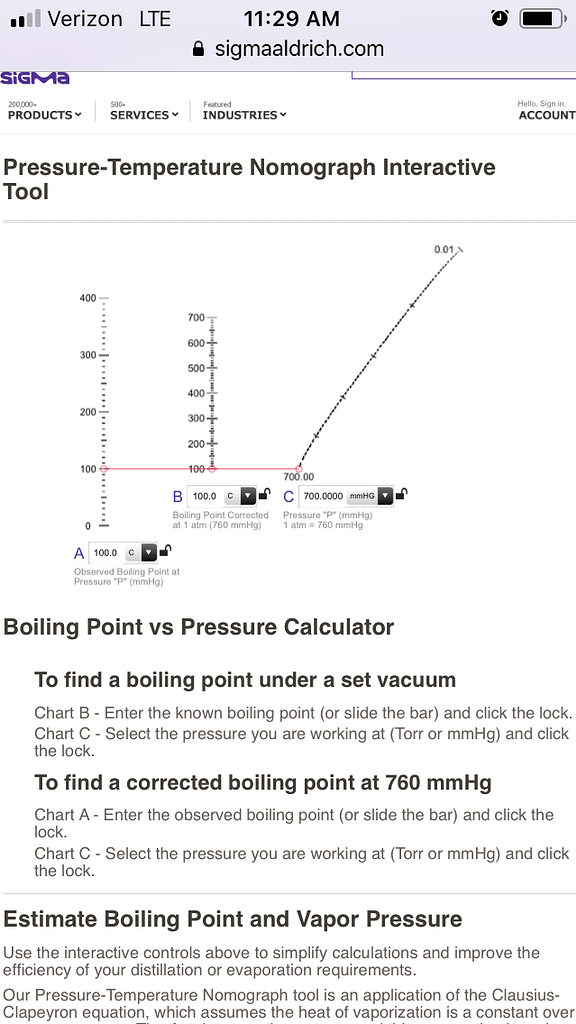
![Nomograph for the calculation of boiling points under vacuum - [www.rhodium.ws] Nomograph for the calculation of boiling points under vacuum - [www.rhodium.ws]](https://erowid.org/archive/rhodium/chemistry/equipment/pictures/nomograph.gif)