Preparation of Al2O3–CeO2 by Hydrothermal Method Supporting Copper Oxide for the Catalytic Oxidation of CO and C3H8 | Industrial & Engineering Chemistry Research
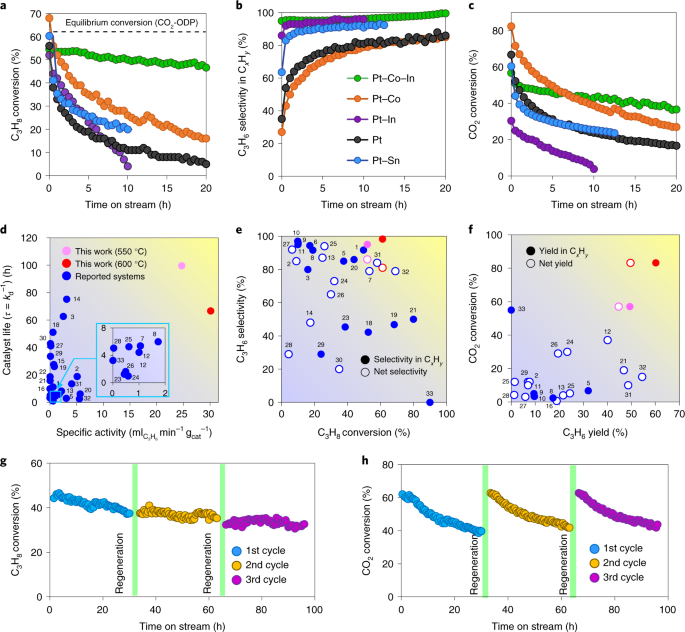
Ternary platinum–cobalt–indium nanoalloy on ceria as a highly efficient catalyst for the oxidative dehydrogenation of propane using CO2 | Nature Catalysis

Low-temperature Cu(I) loading on a mesoporous Metal–Organic framework for adsorptive separation of C3H6/C3H8 mixtures - ScienceDirect

Customized H-bonding acceptor and aperture chemistry within a metal-organic framework for efficient C3H6/C3H8 separation - ScienceDirect

Calculated concentration profiles of C3H8, C3H6, C2H6, C2H4, CH4, and... | Download Scientific Diagram
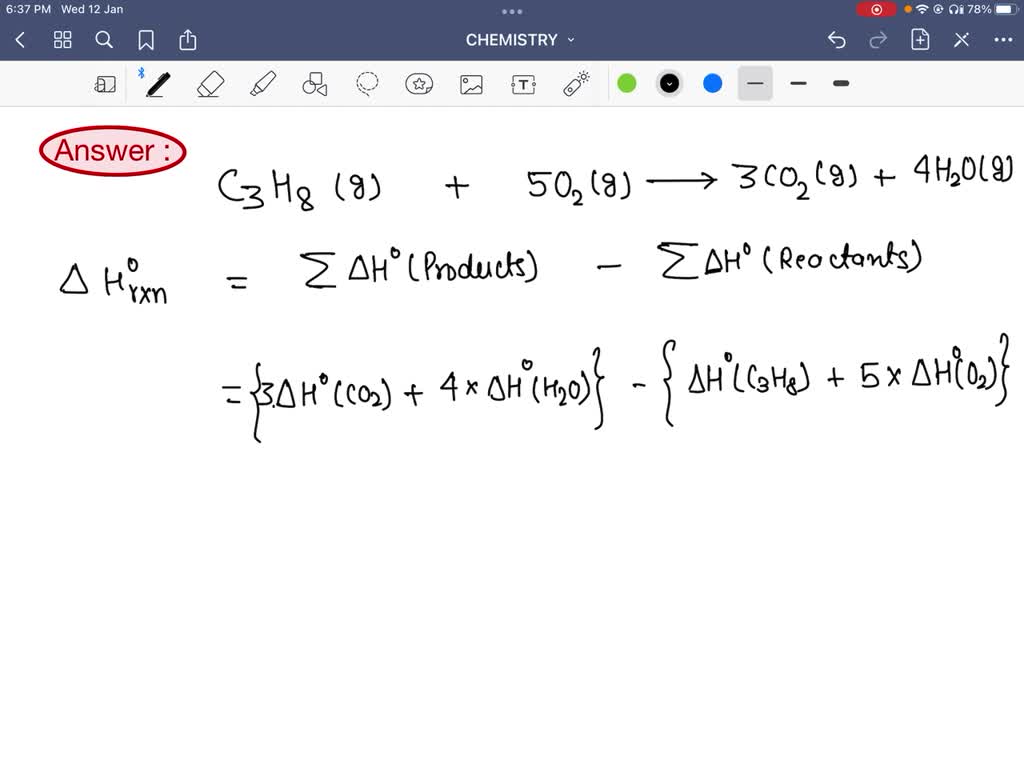
SOLVED: Using the standard molar enthalpies of formation given, calculate the standard enthalpy of reaction for the combustion of propane (C3H8) into carbon dioxide and water: C3H8(g) + 502(g) 3 3C02 +

Oxygen Vacancy-Governed Opposite Catalytic Performance for C3H6 and C3H8 Combustion: The Effect of the Pt Electronic Structure and Chemisorbed Oxygen Species | Environmental Science & Technology

Comparison of OH radicals between DC electric field at ± 3.4 kV and AC... | Download Scientific Diagram

SBS-C3H8 Propane Detector Cut Sheet - Storage Battery Systems, LLC - PDF Catalogs | Technical Documentation | Brochure
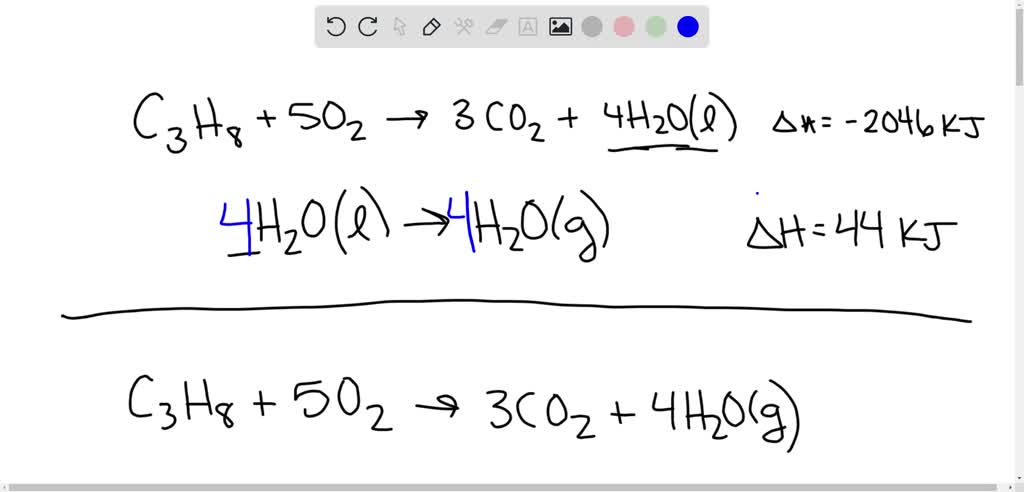
SOLVED: Find ΔH° for the reaction C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(l). ΔH° = -2046 kJ for the reaction: C3H8(g) + 5 O2(g) → 3 CO2(g) + 4

Comparison of OH radicals between DC electric field at ± 3.4 kV and AC... | Download Scientific Diagram

Thermodynamic characterization of the (H2 + C3H8) system significant for the hydrogen economy: Experimental (p, ρ, T) determination and equation-of-state modelling - ScienceDirect

Scheme of the dissociation of C4H10/C3H8 molecules by electron collisions. | Download Scientific Diagram