An I.C. Engine uses 6 kg of fuel having calorific value 44000 kJ/kg in one hour..... | Mechanical Engg Simple Notes ,Solved problems and Videos

Toaz - Good - STEAM TURBINE 1. A steam enters the turbine at 1 Mpa and 320°C. The turbine internal - Studocu
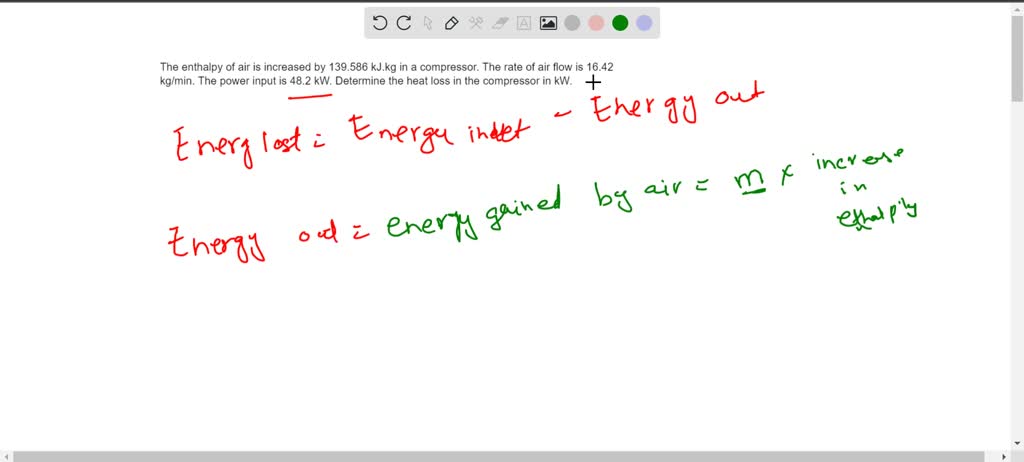
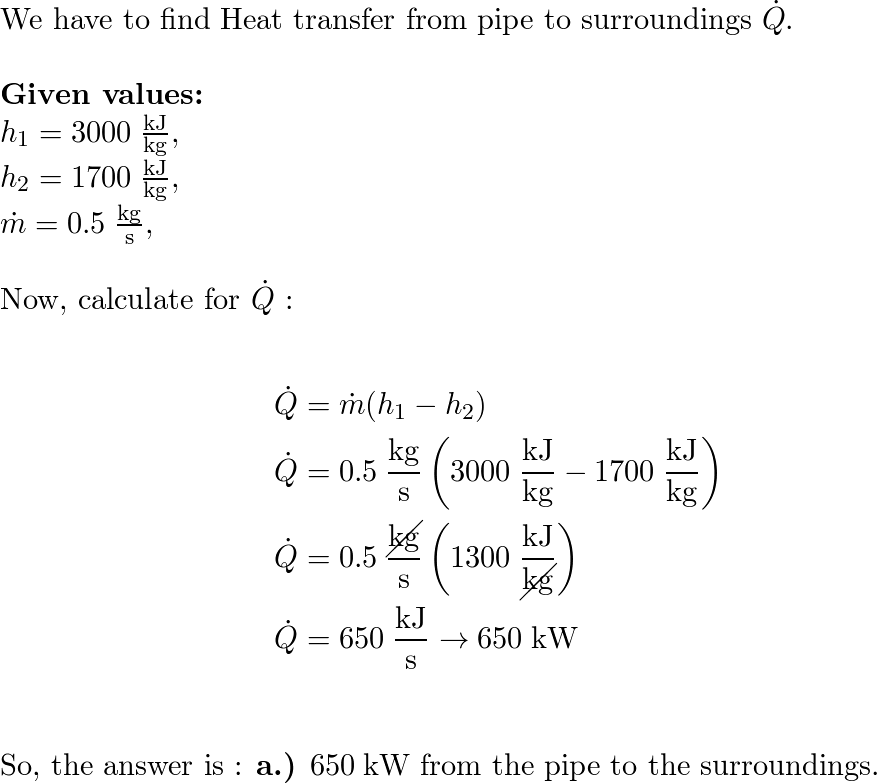
SOLVED: The enthalpy of air is increased by 139.586 kJ.kg in a compressor. The rate of air flow is 16.42 kg/min. The power input is 48.2 kW. Determine the heat loss in
1.0 kg of water is contained in a 1.25 kW kettle. Calculate the time taken for the temperature of water to rise from 25^@C to its boiling point 100^@C. Specific heat capacity
Chapter two Conservation of Energy and The first Law of Thermodynamics Principle of energy conservation:- State that energy can

Helium is to be compressed from 105 kPa and 295 K to 700 kPa and 460K. A heat loss of 15 kJ/kg occurs during the compression process. Neglecting kinetic energy changes, determine

Thermodynamics 1 - KJ kg−k Sact<Ssat:wet¿get x:Sact=sf+xsfg5.2=2+x(4) x=0¿68% 2. At 10m3 vessel - Studocu